
Anna Cifuentes-Rius
Monash University, Australia
Title: Two stage, dual action cancer therapy with targeted porous silicon nanoparticles
Biography
Biography: Anna Cifuentes-Rius
Abstract
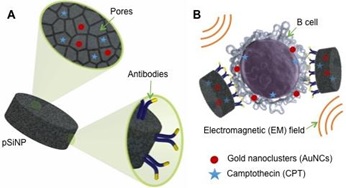
Despite the advances in developing efficient chemotherapy drugs, their efficacy may be diminished by the predisposition of some tumors to drug resistance and non-specific toxicity. Nanomedicine offers the possibility of tackling these key clinical challenges, by designing target delivery platforms for a combination of cancer therapies. To overcome drug resistance, we explore the synergy of hyperthermia with conventional chemotherapy. Due to the higher susceptibility of cancer cells to elevated temperatures compared to healthy cells, hyperthermia stimulates the uptake of anticancer drugs in tumor cells. Thus, nanoparticle (NP)-based delivery systems combining hyperthermia with traditional chemotherapeutics may afford the efficient treatment of highly drug-resistant tumors. Additionally, NP vectorization of therapeutics by actively targeting membrane receptors overexpressed in cancer cells has been recently suggested as a way to ensure selective delivery and improve therapeutic outcomes. Porous silicon (pSi) NPs are (i) biodegradable, (ii) suitable for conjugation with moieties for targeting of a specific cell population, and (iii) exhibit efficient loading of chemotherapy drugs. Here, we utilized these unique characteristics of pSiNPs and loaded them with multiple therapeutics while also immobilizing cell-specific antibodies to achieve active targeting. We have developed antibody functionalized pSi NP loaded with a combination of chemotherapy drug and gold nanoclusters (AuNCs). By selective targeting, these nanocarriers were observed to actively deliver both the chemotherapy drug and AuNCs to human B cells. The accumulation of AuNCs to target cells rendered them more susceptible to the co-delivered chemotherapy drug when an external electromagnetic field in the microwave region was applied. This approach represents a targeted two-stage delivery nanovector that takes advantage of dual therapeutic action in order to enhance cytotoxicity.
Recent Publications:
1. H Alhmoud, B Delalat, R Elnathan, A Cifuentes-Rius, A Chaix, ML Rogers, JO Durand and NH Voelcker (2015) Title of the abstract. Advanced Functional Materials 25:1137-1145.

